In part 1, we looked at how DNA in our cells can cause cascades of effects that eventually show up as observable traits, like hair color or sickle cell anemia. As an example, we looked at how a specific group of letters in some people’s DNA can lead, through a series of steps, to those people having blue eyes:
Specific HERC2 gene in our DNA → rare HERC2 protein → fewer P proteins → less tyrosine → less melanin → bluer eyes
We gave some pretty straightforward explanations for each link in the chain above, except for the first step of how we go from a gene in someone’s DNA to a protein in their body. This time, we’re going to start to talk about that step.
Why DNA?
A lot of questions spring to mind immediately. Probably the most immediate one is: how do we know that DNA is important at all? Why do we need that first step to get from DNA to protein? Maybe DNA is just superfluous, and we can get away with just having the protein, without even having to worry about DNA. How do we even know DNA exists in our bodies in the first place?
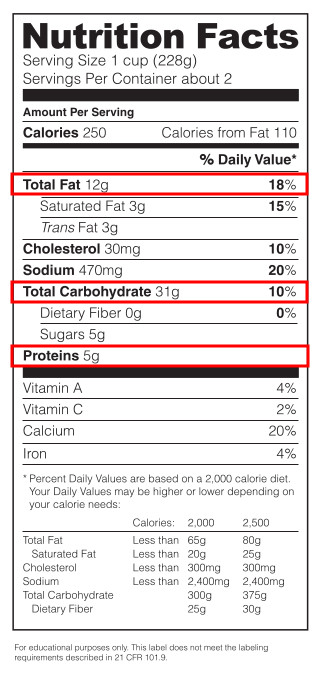
As the Age of Enlightenment swept over Europe, it logically followed that people would start mashing and crushing up living things, because that’s what enlightened people do. As a result, they began to realize that living matter was made up of different kinds of chemicals, and that these chemicals could be characterized based on what they dissolved in. For instance, most of the crushed up material could be dissolved in water, but some of it couldn’t, and had to be dissolved in alcohol or ether instead. Some of the water-dissolved material could be reacted with saliva, and some of it could be reacted with stomach secretions (scientists typically used secretions from a pig’s stomach–don’t ask me how they got it out of the pig). The water-soluble material that reacted with saliva were later identified to be carbohydrates, and the stuff that reacted with stomach secretions were identified as proteins. The stuff that dissolved in alcohol or ether was mainly fats, or lipids. Most living matter is made up of these three groups: carbohydrates, proteins, and fats; a fact that is still reflected on nutrition labels on our foods.
At the same time, a lot of scientists were busy peering into microscopes and discovering that a whole invisible world existed all around them. In 1833, Scottish botanist Robert Brown (yes, the same guy that discovered Brownian motion) noticed that a dark spot was always present in the orchid cells he was examining. He at first called the spot an areola, but then presumably thought better of it and subsequently dubbed it a “nucleus.” A multitude of other scientists, including Theodor Schwann, noticed the same rough structures in animal embryos and began to put together the cell theory of life: that all living creatures (plants and animals) are made up of cells, the most obvious structure of which is the nucleus. (An interesting aside: Schwann was also the first to isolate the component of stomach secretions mentioned above that reacts with proteins. He called it “pepsin,” and it is one of the most important enzymes in our body for digestion).
By the 1860’s, the cell theory had become well-accepted by most biologists, and chemists were proceeding apace with the characterization of the chemicals that make up living matter. In the midst of this, Friedrich Miescher, a young German biochemist (though the word “biochemist” wouldn’t exist for another 70 years), decided he was going to try to classify all the matter in living cells. He wanted to focus on proteins, because at the time it was believed that proteins were the most important part of living matter. However, he quickly noticed that another, as yet unclassified material kept mucking up all of his experiments. He believed that this material was coming from the nuclei of the cells he was working with, and set about isolating it.
Now, up to this point no one had ever separated nuclei from their host cells and had the nuclei stay intact, so Miescher spent a lot of time on trial and error experiments trying to get rid of the outer parts of cells without annihilating the nuclei. He chose leucocytes (white blood cells, pronounced “luke-o-sites”) as his go-to cells; he got them off pus-soaked bandages, which were in plentiful supply on account of his lab’s proximity to a surgical clinic in Tübingen. Over the course of several months and more soiled surgical bandages than anyone cares to think about, Miescher’s efforts converged on an optimized method for getting nuclei out of cells unharmed, and extracting the substances waiting for discovery within. Here’s a brief rundown of how he did it:
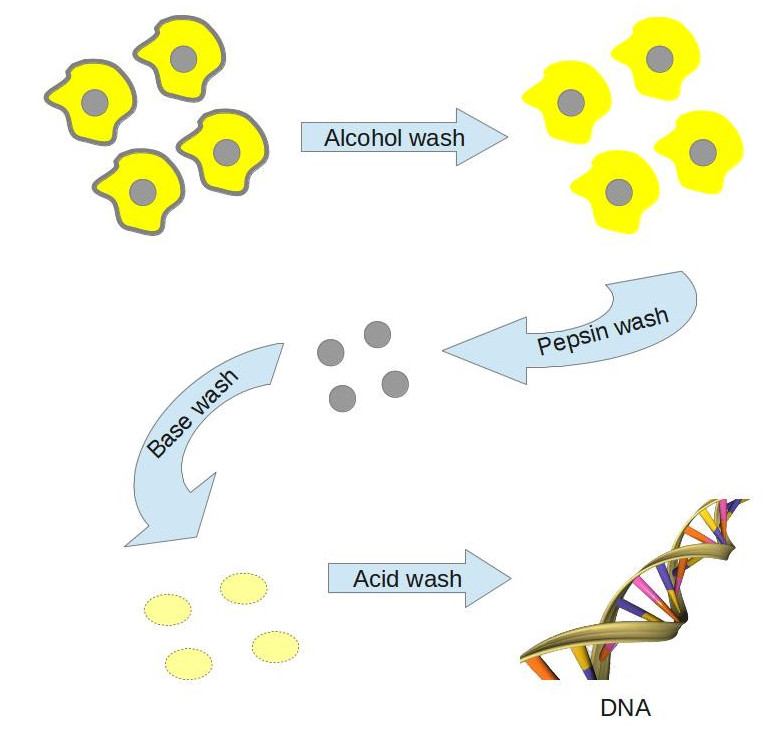
We mentioned earlier that alcohol and pepsin from pig stomachs can dissolve away some parts of living matter, and that’s exactly what Miescher did in the first two steps of isolating DNA: he washed the cells with alcohol to get rid of the outer cell membrane (consisting mostly of fats), and then digested the cells in pepsin solution over a number of days to take off the remaining material and leave the nucleus. At this point, he noticed that if he put the nuclei in a solution of sodium bicarbonate (baking soda), the nuclei would swell up and become translucent. If he then took this material and neutralized it with acid, he got a solid material. What’s going on here? Since Miescher was a chemist, he understood that if something dissolves in a base (such as baking soda) but resolidifies, or precipitates, when acid is added, then that substance is most likely an acid. In fact other scientists would call his discovery “nucleic acid” (the “NA” in “DNA”). A full explanation of how Miescher knew that acids dissolve in bases but not acids will have to wait for a future HDWKI. For now, suffice it to say that when the nuclei were put into a base solution, the solution dissolved the DNA inside the nuclei, allowing it to slip out through the membrane. Once the membranes were filtered away and the solution was re-acidified, the DNA came crashing out of solution and Miescher was able to isolate it and do subsequent experiments on it.
I’ll go into much more detail about DNA in future posts, but we can look at some of the results of Miescher’s first experiments right now. His work revealed some fundamental facts about DNA that most of us today take for granted. For one, he repeated the DNA isolation with many different types of cells, showing that almost every cell, plant and animal, contains DNA. He showed that, when dissolved, DNA diffused through the solution very slowly; he took this to mean that DNA must be a particularly large molecule (although he couldn’t have fathomed how large–modern values of DNA’s size are thousands of times larger than Miescher’s estimate). He showed that DNA did not dissolve in pepsin solution, and therefore was not a protein. He also burned the DNA to analyze what elements it was made up of. He showed that it was mostly carbon, hydrogen, nitrogen, and oxygen–this is also true of proteins. However, the final nail in the coffin for the protein theory of DNA was the discovery that, while proteins were known to contain sulfur and no phosphorus, DNA was found to contain phosphorus but no sulfur. How did he know this? How did he glean this information just by burning DNA?
Elemental analysis or: How I learned to stop worrying and love the combustion analyzer
When scientists attempt to figure out what elements make up a substance, they use a technique called elemental analysis (scientists aren’t the most imaginative bunch). Basically, they burn things and see what happens to them. When you burn something, you’re really just reacting that substance with oxygen (we’ll talk about this in a future HDWKI). When you burn living matter–or rather, matter that used to be living–you generally get three types of gases: carbon dioxide (formed when carbon reacts with oxygen), water vapor (formed when hydrogen reacts with oxygen), and various nitrogen oxides (formed when nitrogen reacts with oxygen). If you burn proteins, you also get a small amount of sulfur oxides (also called sulfates), and, as Miescher found, if you burn DNA, you get a small amount of phosphorus oxides (also called phosphates).
That’s all fine, but how do we know that we get those gases? How do we prove it? Well, the easiest one to prove is probably the water vapor. If you burn something, collect the gas that comes off, and then cool it down, the water vapor will condense into liquid water. You can actually measure how much water condenses and it’ll give you a pretty good idea of how much hydrogen you started with. Alright, one gas down. Detecting the rest of the gases involves bubbling them through water. We’ve already talked about what happens when you bubble carbon dioxide through water (long story short, you get soda water). When nitrogen, sulfur, and phosphorus oxides come into contact with water, you get nitric acid, sulfuric acid, and phosphoric acid. With nitrogen, the procedure is even easier: just look at the gas. Nitrogen dioxide is a reddish brown colored gas and has a very distinctive smell (kind of like bleach…btw, please don’t go smelling nitrogen oxides; they’re pretty poisonous):

Okay, so now you’ve condensed the water out of your gases, passed the carbon dioxide through water to make club soda, and seen reddish vapors indicating nitrogen oxides. You can pass the sulfates and phosphates through water to make sulfuric and phosphoric acid. But wait, how do you know whether you’ve made sulfuric or phosphoric acid? After all, they both look alike: there’s really no outward difference between the two. What distinctions can be made?
Nowadays, we have many different methods at our disposal to determine the elemental makeup of a substance; however, in the 19th century, Miescher really only had one. It was a holdover from the alchemists of the middle ages and it had to do with finding out which substances were soluble in what solutions. The classic method for determining sulfur content was to bubble the gas through a solution of barium chloride. If there were sulfates, then an insoluble white powder (barium sulfate) would begin to form in the liquid immediately. A similar procedure could be undertaken for phosphates, this time using a solution of ammonia and molybdenum. In fact, this method of phosphate detection is still used by environmental agencies to analyze the presence of phosphorus-rich fertilizer in runoff and groundwater. In either case, the amount of powder formed will tell you how much sulfur or phosphorus you started with.
So, using the techniques above, Miescher was able to show definitively that DNA was not a protein, but rather a completely new substance, and he was able to characterize it in pretty good detail. But to do all this would have required thousands of surgical bandages full of pus, right? Well, that’s where Miescher got creative. It just so happened that the annual salmon migration upriver passed right by his lab. So, this being the Age of Enlightenment and all, he did what any enlightened person would do and began collecting as much salmon sperm as he possibly could. This was actually a genius move, because sperm cells (other than their tails) are almost entirely nucleus.
The use of sperm cells allowed Miescher to amass huge quantities of relatively pure DNA and carry out many of the experiments I mentioned above. Alas, the siren song of the salmon sperm eventually must’ve gotten to him, as he eventually turned his research attention to physiological changes to salmon during their mating season. One biography describes his “seminal contributions” to salmon gonad development science. You can’t make this stuff up. Miescher would never again contribute meaningfully to DNA research.
Next time, we’ll look at how scientists figured out that DNA played a role in heredity, the passing on of genetic traits from parent to child. We’ll discuss how DNA grew in importance with each subsequent experiment, ultimately leading to the discovery of a code buried within the chains of DNA molecules, and then we’ll crack that code. Stay tuned.
References
The early history of cell biology is discussed in:
- Williams, Henry Smith, 1863-1943. A History of Science: in Five Volumes. Volume IV: Modern Development of the Chemical and Biological Sciences
specifically chapter 5, which can be found at:
Details from Friedrich Miescher’s life are discussed in:
- Dahm, Ralf. “Friedrich Miescher and the discovery of DNA.” Developmental Biology. 2005, Vol. 278, p. 274.
and references therein, especially:
- W. His (Ed.) et al., Die Histochemischen und Physiologischen Arbeiten von Friedrich Miescher (sorry, it’s in German)
Everything you could ever possibly want to know about elemental analysis can be found in this book:
- Grasshoff, K., K. Kremling, and M. Ehrhardt. 1999. Methods of seawater analysis, 3rd ed., Wiley-VCH , New York